Why do electrons orbit the nucleus without falling into it?
Electrons do not fall into the nucleus because of quantum energy levels and the balance of electromagnetic forces.
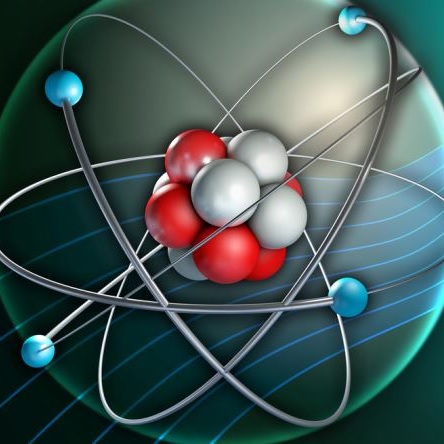

Electrons do not fall into the nucleus because of quantum energy levels and the balance of electromagnetic forces.


Sound cannot travel in a vacuum because there is no matter to transmit vibrations or carry the energy.
Read More
The question of why time flows in one direction is explained by physics and entropy because the universe moves toward increasing disorder.
Read More
The sky appears blue due to Rayleigh scattering of sunlight in the atmosphere, while weather conditions and particles alter its shades, as seen during sunset.
Read More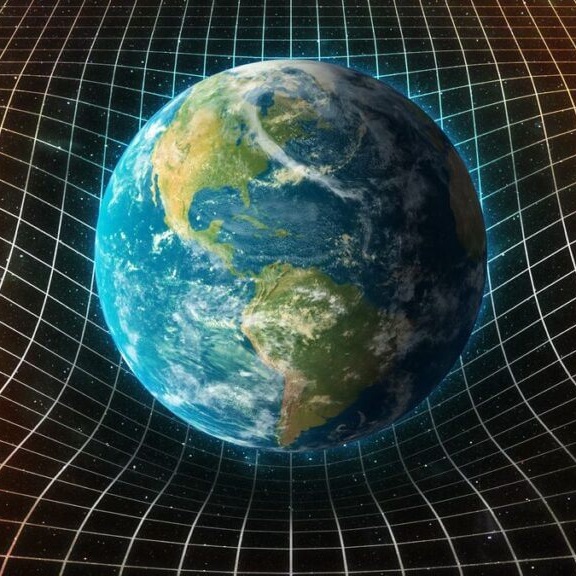
Gravity exists because mass bends spacetime, pulling objects toward each other.
Read More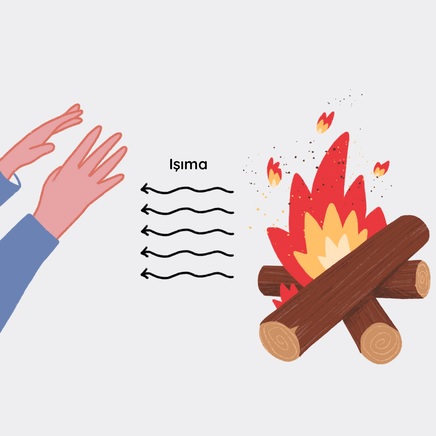
Heat always flows from hot to cold because energy balance is governed by the second law of thermodynamics.
Read More
The mass of matter does not change because atoms are conserved and energy does not alter matter itself.
Read More